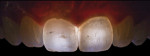
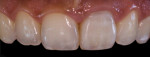
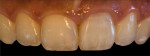
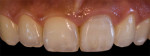
Digital Photographic Procedure for Comprehensive Two-Dimensional Tooth Shade Analysis
Yiannis Brokos, DDS, MSc, Dr. med. dent.; Minos Stavridakis, DDS, MSc, Dr. med. dent.; and Ivo Krejci, Prof. Dr. med. dent.
Abstract
Current commercially available restorative materials vary in their esthetic properties, depending on brand and shade. Variations are related not only to basic color parameters such as hue, chroma, and value, but also to other important properties that affect the overall esthetic restorative outcome, such as opalescence, fluorescence, translucency, and metamerism. Fluorescence and bluish opalescence, though associated with the ingredients and chemical composition of the material, may be controlled and refined by a proper layering technique if that pretreatment analysis has been performed with the aid of appropriate photographic techniques. Digital cameras and dental photography have long been imperative tools for clinicians in their daily practice. Traditionally, digital photography has been used for recordkeeping, documentation, presentation, and informing patients of their oral status before and after treatment. Today, evolved techniques facilitate clinicians’ ability to compare the esthetic properties of restorative materials with those of natural teeth for delivery of natural-looking restorations. Moreover, documentation obtained before and after restoration may be used for more comprehensive information for the patient.
Shade matching between natural teeth and composite materials is critical when creating esthetic restorations.1-6 Hue, chroma, and value are basic color parameters that influence the esthetic outcome of a tooth-colored restoration.7-13 The shade of contemporary enamel and dentin composite materials should, and most likely do, accurately mimic the shade of enamel14-17 and dentin.18,19 Unfortunately, commercially available composite materials, even of the same shade, vary in fundamental properties, such as opalescence,20-24 fluorescence,25-33 translucency34-48 and metamerism49,50, between different brands and shades. Clinicians should be able to determine the variations of these properties and either correct any mismatch in the course of the layering technique51-58 or at least keep a record of non-correctable differences. The application of appropriate digital photography techniques is the procedure of choice to control and document the esthetic properties of current materials.
Digital cameras have undergone impressive evolution since their development in the early 2000s59,60 incorporating emerging technologies to make photography highly accurate, simple, and economic. Digital photography61-65 has long been a necessary tool in dentistry for recording, diagnosing, communicating, presenting, informing, and documenting66,67 the oral status of the patient before and after treatment. A digital single lens reflex (DSLR) system should be considered standard equipment for dental photography. A DSLR camera body using an 80-mm to 105-mm macro lens and a ring flash or a twin flash system is the basic setup for capturing detailed extraoral and intraoral images. Most current digital camera bodies are efficient enough in terms of resolution, aperture, and exposure settings. ISO configuration is considered an important and sensitive parameter to be investigated, especially for advanced fluorescence, red-orange opalescence, and translucency recording. Macro lenses of 80 mm to 105 mm provide an ideal combination of proper magnification within convenient working distance for intraoral close-up photography. A life-size reproduction of the photographed object is referred to as 1:1 magnification and, in frontal dental photography, comprises the four maxillary incisors. Electronic flashlight is necessary for proper illumination of the dark areas within the intraoral environment. The two main types of flash systems for dental purposes are a ring flash and a twin-light flash. The light of a ring flash eliminates shadows in the oral cavity but may produce undesirable specular reflections. The illumination by a twin flash produces a soft and 3-dimensional lighting effect with prominent and detailed surface morphology.
Evolved capturing techniques68-70 enable clinicians to observe the physical characteristics and properties of natural dentition. Surface details, such as macro- and micromorphology, texture, luster, gloss, enamel cracks and striations, chromatic mapping, and dentinal architecture, can be revealed and recorded with direct reflective lighting techniques using different illumination angles. In addition, photographic applications for daily clinical practice have recently been developed to address the refinement or diagnosis of material properties, such as opalescence, fluorescence, translucency, and metamerism.
The purpose of this article is to explore through the eye of the lens these optical properties, while demystifying and describing the respective photographic applications from a clinical and technical point of view.
Photographic Equipment
DSLR Camera, Lens, and Flash
A DSLR Canon 550D camera (Canon U.S.A., Inc., usa.canon.com) with a Canon 100-mm macro lens and a Canon MT-24EX twin-light flash were used in the photographic protocol presented. A custom-fabricated plastic o-ring, with four metallic screws at 0o, 90o, 180o, and 270o, was fixed with silicone to the original flash framework, ready to receive the interchangeable add-on filters (Figure 1 and Figure 2).
Flash Plastic Diffusers
The full excitation wavelength range of the xenon flash lamps is between 300 nm and 800 nm. When the original protective plastic diffusers cover the flash lamps, the range is limited within the visible light of 400 nm to 700 nm. In the case of fluorescence, the appropriate excitation wavelength is in the ultraviolet (UV) range, more precisely at 365 nm. Both plastic diffusers were removed from the flash and attached to the interchangeable plastic framework (Figure 3 and Figure 4) to protect mechanically both flash lamps and to protect the polarizing membrane (described later) from the increased output of the lamps. For the cross-polarizing add-on filter, the framework with the diffusers and the framework with the polarizing membranes were combined (Figure 5). Additional caution was taken by placing clear plastics in place of plastic diffusers.
Three interchangeable plastic frameworks with different filters were fabricated and connected to the underlying flash by four magnets, either separately or combined together, in front of the lens and the flash lamps. The first contained the diffusers (Figure 6), the second the polarizing membranes (Figure 7), and the third the 365-nm UV glass filters (Figure 8). Removing the plastic diffusers from the flash may lead to flash warranty loss. Because the purpose of this article is to present the principal technical aspects of the applications, the warranty was not taken into consideration.
Cross-Polarized Filters
Two pieces of a polarized plastic membrane were placed in parallel on both sides of the plastic framework. Another piece was placed in the center, perpendicularly to the lateral pieces (Figure 9). The cross-polarized filter may not be used directly on the flash lamps because the membrane might be burned by the energy of the flashes (Figure 10). For this reason, as mentioned previously, the cross-polarized filters were placed on top of the add-on frame with the plastic diffusers.
365-nm UV Filters
The fluorescence filters were composed of two 365-nm UV glass filters placed on both sides of the plastic framework to cover the flash lamps. No additional filter was required in front of the lens (Figure 11).
Setup for Metamerism
For metamerism diagnosis, a device with continuous illumination from two different light-emitting diodes (LEDs) (Rite Lite 2™, AdDent, Inc., addent.com) generating three different illumination qualities, such as 5500°K simulating day light, 3200°K simulating incandescent light, and 3900°K simulating fluorescent tube light, was luted to a plastic o-ring with four magnets (Figure 12). In this way, it was possible to attach it in front of the lens for static images (Figure 13) without flash.
The color rendering index (CRI) is described as the relative ability of a light source to replicate and is reported as a number between 0 and 100. A CRI score of 100 would accurately reproduce the colors on a sunny day at noon. This lighting condition is considered the ideal illumination environment for shade matching in restorative dentistry, even though it is rarely present during shade matching.
Rite Lite 2 has LEDs with high CRI values, varying from 87 to 92, thus approaching the ideal illumination conditions (CRI 100, 5000°K to 6000°K).
Fluorescence
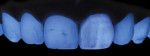
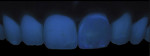
Fluorescence71-76 is a variation of luminescence. The more fluorescent a material is, the more bright and ‘vital’ it appears. Fluorescence is defined as the ability of a natural or artificial substance to emit visible light spontaneously when irradiated by UVA illumination. The excitation spectrum of dentin has a center wavelength of 365 nm, and the fluorescence emission peak is observed at 440 nm with a full width at half maximum of 20 nm. Enamel presents a much less intense fluorescence peak at 450 nm, which slowly decreases up to 680 nm. Thus a wide, but not intense, band of fluorescence spectrum is present in enamel. Studies show that dentin is three times more fluorescent than enamel, and that dentin fluorescence intensity increases over time because dentin has higher quantities of minerals, pyrimidine, pyridinoline, tryptophan, and hydroxypyridium.77-82
Photographic Application
Fluorescence can be captured with two photographic applications using continuous or flash lighting. The traditional method of continuous lighting is quite complicated. It requires a continuous UV light source and a dark room to avoid any other artificial lighting source in the operatory field to make the light visible because the intensity of the fluorescence is very low. In addition, the photographic recording necessitates long exposure times of several seconds and increased ISO sensitivity of the sensor of the DSLR camera with the disadvantage of increased picture noise, the need for stabilization of the camera on a tripod, and significant irradiation of the patient by UV light (Figure 14).
Recently, the authors proposed a novel setup that avoids all these practical disadvantages and minimizes the exposure of the patient to UVA light. This setup consists of an interchangeable UVA 365-nm excitation filter placed in front of the commercial macro flash lamps after removal of their plastic diffusers, together with a DSLR camera with a macro lens. This allows for fluorescence documentation under normal dental office conditions in the same way as standard clinical photography using a macro lens and a flash does, without the need of a dark room or extended exposure times (Figure 15).
Clinical Significance
Fluorescence is considered a clinically significant optical property in esthetic restorations because, under fluorescence, teeth appear more vital, whiter, and brighter. Under black lighting, such as in nightclubs, where UV-coated lamps emit the appropriate excitation light to induce fluorescence, restorations should not be distinguishable from the natural dentition. A perfectly integrated esthetic restoration should exhibit a similar fluorescence to that of the natural dentition, and the practitioner must be able to check on this property in his or her routine clinical setting. Enamel and dentin composite materials should closely mimic the fluorescence emission levels of enamel and dentin. Unfortunately, currently commercially available composite materials vary in their fluorescence, depending on brand and shade.83-85
Opalescence
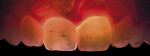
Opalescence86,87 is defined as the optical property observed in natural tissues or artificial substances to appear bluish-greyish in reflective illumination (opalescent halo) and yellowish-orange-brown under transmitted illumination (counter opalescence). The enamel of natural teeth is opalescent. This optical phenomenon is based on the difference in refractive indexes of the enamel components, which are hydroxyapatite crystals and water. Moreover, the specific dimension and diverse orientation of the hydroxyapatite crystals scatter light within the visible spectrum, more in short wavelengths for bluish effect and less in long wavelengths for yellowish-red effect. These effects are clearly visible, especially at the incisal enamel edge and also on the border between enamel and dentinal lobes.
Photographic Application for Yellowish-red Opalescence
To document and photograph yellowish-red opalescence, transmitted continuous light must be used for illumination, without flashlight. An appropriate illumination source for this application is a white LED in the oral cavity directed toward the palatal surface of the tooth to be examined. The exposure time is increased to 1/60 (but not below, to avoid a shaking effect), the aperture is decreased to around f = 10, and the sensitivity of the sensor is increased to a high value (~ 3200). With these settings, the DSLR camera is sufficiently sensitive to capture enough light without any further increase in the exposure time, which would necessitate a tripod to avoid shaking (Figure 16). The LED Microlux™ Transilluminator (AdDent) with a 3-mm microtip glass light guide was used for the specific photographic application.
Photographic Application for Bluish Opalescence
To capture bluish opalescence, reflective technique is acquired using normal macro photography and flash lighting. The use of a polarizing filter is helpful, eliminating whitish areas of specular reflections and revealing the exact anatomy of the opalescence zone (Figure 17).
Clinical Significance
Ideal restorative materials should exhibit comparable properties to natural dentition. Unfortunately, commercially available composite materials, as they do for other optical properties, vary in their opalescence. Bluish opalescence, which is more evident than yellowish-red opalescence in social settings, may be determined with the appropriate photographic technique and controlled at the restorative phase.
Translucency
Translucency is the property of a material that allows for light transmission but also dissipates the light within the material.88-90. As such the material is not completely transparent but has an appearance of a milky glass. In other words, translucency is the relative amount of light transmittance or diffuse reflectance from a material.91Factors that influence the transmission of light within composite resins are related to structural components, such as the resin matrix and filler contents, the difference of refraction indexes between them, the size and shape of inorganic fillers, and, finally, pigments and other additives. The aging of the material and the polymerization procedure used may also influence the degree of translucency that current composite materials exhibit.
Photographic Application
To document and photograph translucency, a similar method to the capturing of yellowish-red opalescence is necessary. Thus, transmitted continuous light for illumination, without flash light but in contact with the palatal surface of the tooth to be examined, is required (Figure 18).
Clinical Significance
Dental enamel is translucent. Consequently, contemporary composite systems have highlighted the importance of this property. Translucency of enamel materials provides a depth of color for the underlying dentin and contributes to shade matching by enhancing the chameleon effect. Enamel becomes more translucent with age.
Metamerism
The metameric effect leads to different color changes under different lighting conditions.92-97 Two substances that have the same color appearance under certain lightning conditions may have different color appearances when the lightning conditions change. This fundamental effect in the field of restorative dentistry may be influenced by three parameters: the material, the observer, and the lighting conditions. For example, if the spectrophotometric light emission curve of the material in the visible light range is different from the one of the surrounding tissue, then metamerism is observed. This parameter can only be controlled if the curve of the material is known and compared before its use. Regarding the observer parameter, individual subjective color perception of the observer may be biased. This could be due to deuteranopia, for example. Color vision deficiencies by practitioners, especially men, who are much more likely than women to have deuteranopia, should therefore be considered. Finally, daylight in outdoor environments and room and mixed lighting conditions in various indoor environments may influence the appearance of restorations because of metamerism. If composite restorations exhibit a coherent appearance and shade matching under these lighting conditions, then they are considered to have successfully overcome the effect of metamerism.
Photographic Application
Commercially available devices using LED technology can simulate multiple lighting conditions, which may be used for the disclosure of metamerism. One of these devices was adapted in front of the lens of the digital camera to capture images in three different color temperatures (Figure 19 through Figure 21), disclosing shade differences if metameric effect was present. Figure 22 depicts how the restorations appeared under daylight, Figure 23 under incandescent light, and Figure 24 under ambient light.
Clinical Significance
A successful restorative outcome should be evaluated under varying lighting conditions that are representative of the social environments and activities of the patient. Shade matching procedures, therefore, should be performed under daylight close to a window, as well as under room or ambient light in the operating environment.
Value
Value is one of the three basic color parameters and is related to brightness (ie, to the degree of white and black) (Figure 25).
Photographic Application
The color of a cross-polarized photograph is converted to a gray scale image by using a generic software program, such as Adobe Photoshop®.
Clinical Significance
The value of the restoration may be comparatively determined during the shade matching procedure and corrected if necessary within the restorative phase.
Chromatic Charting or Mapping
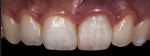
Shade matching98-104 between natural teeth and composite materials is the primary objective when creating direct restorations. This involves shade or chromatic mapping, which aims at identification of shade distribution, revealing important color information in two dimensions. Identifying dentin areas of diverse gradation in hue and chroma, the configuration and architecture of the lobes, bluish opalescence, and regions of intense hue are related to this.
Photographic Application
Cross-polarized reflective capturing technique is considered the application of choice for chromatic mapping. The cross-polarized technique was described for both analog and digital cameras many years ago.105 Polarized photographs eliminate specular reflections of the flash, which makes identifying shade distribution easier. The photographic equipment is the same (camera body, macro lens, macro flash), but an additional polarizing filtering system is needed. A polarizing membrane sheet is placed in front of the flash lamp, covering the surface. For both the ring flash and twin light, the piece of membrane is split in two to cover the left and right flash lamps, always maintaining the orientation of the sheets parallel to each other. Another piece is placed perpendicular to the other sheets in front of the lens. In this way, the pieces are cross-polarized against each other, creating a matte photographic image (Figure 26).
Clinical Significance
Accurate chromatic mapping is an essential step for the overall successful shade matching between natural teeth and composite materials.
Discussion
The present clinical report describes a comprehensive approach in documenting the optical properties of both dental restorative materials and natural hard dental tissues. Based on this concept, a simple and straightforward procedure is outlined and may be used during the restorative phase. In this way, documentation can take place in three different yet equally important time intervals: before, during, and after the restorative session. In direct adhesive procedures, such a concept is useful in refining the optical properties of the restorative materials, increasing the esthetic success rate of the restorative procedure.
Clinicians generally are familiar with the equipment and basic photographic techniques and are relatively confident working with digital cameras and dental photography. Updating the indications, optimizing the use of existing equipment, and decreasing overall costs were among the main goals of the authors. The photographic applications are explained in detail and clinicians can use them with any digital camera and flash system. Commercially available polarizing membrane sheets (linear polarizing film sheet) are very inexpensive and ideally suited for the cross-polarizing application. UVA 365-nm glass filters (eg, Schott® Optical Filters, us.schott.com) of 2-mm thickness are available in the market and are indicated for the recording of fluorescence. LED sources for transillumination (eg, Microlux) and LED illumination devices with different light temperatures (eg, Rite Lite 2) are available in the market and may be used for metamerism, translucency, and red-yellowish opalescence. The latter LED device, even though initially intended for clinical application only, can be easily adjusted in front of the digital camera lens for static no-flash pictures.
The described protocol of comprehensive documentation of the optical properties aims to determine differences between composite materials and natural tissues. Practitioners should be able to expand their knowledge of the shade-matching process and learn to distinguish the color elements that can and cannot be controlled during the restorative phase. Hue, chroma, metamerism, translucency, and red opalescence are inherent properties of restorative materials that may be diagnosed but which may not be influenced during the fabrication of direct restorations when a given brand of a composite material is used. On the other hand, value, bluish opalescence, and fluorescence are all considered inherent properties of the restorative materials that may be diagnosed and refined during the layering of materials in direct restorative procedures.
Conclusion
Within the limits of this clinical report, some conclusions can be drawn. Comparative level of luminosity, fluorescence, opalescence, translucency, and metamerism between natural tissues and dental materials may be recorded in daily practice by any clinician by using low-cost equipment based on a simple DSLR camera combined with inexpensive, commercially available add-on equipment. For direct restorations particularly, color elements that influence the overall restorative outcome, such as luminosity, fluorescence, and bluish opalescence, may be optimized because of correct diagnosis during the layering technique.
About the Authors
Yiannis Brokos, DDS, MSc, Dr. med. dent.
Division of Cariology and Endodontology
University Clinics of Dental Medicine
University of Geneva
Geneva, Switzerland
Private Practice
Rhodes, Greece
Minos Stavridakis, DDS, MSc, Dr. med. dent.
Division of Cariology and Endodontology
University Clinics of Dental Medicine
University of Geneva
Geneva, Switzerland
Private Practice
Athens, Greece
Ivo Krejci, Prof. Dr. med. dent.
Chairman and Professor
Division of Cariology and Endodontology
University Clinics of Dental Medicine
University of Geneva
Geneva, Switzerland
References
1. Miller LL. Shade matching. J Esthet Dent. 1993;5(4):143-153.
2. Tripodakis AP. Shade selection in fixed prosthodontics. Odontostomatol Proodos. 1989;43(6):539-548.
3. Okubo SR, Kanawati A, Richards MW, Childress S. Evaluation of visual and instrument shade matching. J Prosthet Dent. 1998;80(6):642-648.
4. Lagouvardos PE, Diamanti H, Polyzois G. Effect of individual shades on reliability and validity of observers in color matching. Eur J Prosthodont Restor Dent. 2004;12(2):51-56.
5. Clary JA, Ontiveros JC, Cron SG, Paravina RD. Influence of light source, polarization, education, and training on shade matching quality. J Prosthet Dent. 2016;116(1):91-97.
6. Pecho OE, Pérez MM, Ghinea R, Della Bona A. Lightness, chroma and hue differences on visual shade matching. Dent Mater. 2016;32(11):1362-1373.
7. Clark EB. An analysis of tooth color. J Dent Am Assoc. 1931;18(11):2093-2103.
8. Saleski CG. Color, light, and shade matching. J Prosthet Dent. 1972;27(3):263-268.
9. Sproull RC. Color matching in dentistry. II. Practical applications of the organization of color. J Prosthet Dent. 1973;29(5):556-566.
10. ten Bosch JJ, Coops JC. Tooth color and reflectance as related to light scattering and enamel hardness. J Den Res. 1995;74(1):374-380.
12. Sproull RC. Color matching in dentistry. Part I. The three-dimensional nature of color. 1973. J Prosthet Dent. 2001;86(5):453-457.
13. Joiner A. Tooth colour: a review of the literature. J Dent. 2004;32(suppl 1):3-12.
14. Risnes S, Peterkova R, Lesot H. Distribution and structure of dental enamel in incisors of Tabby mice. Arch Oral Biol. 2005;50(2):181-184.
15. He LH, Swain MV. Understanding the mechanical behaviour of human enamel from its structural and compositional characteristics. J Mech Behav Biomed Mater. 2008;1(1):18-29.
16. Myoung S, Lee J, Constantino P, et al. Morphology and fracture of enamel. J Biomech. 2009;42(12):1947-1951.
17. Li R, Ma X, Liang S, et al. Optical properties of enamel and translucent composites by diffuse reflectance measurements. J Dent. 2012;40(suppl 1):e40-e47.
18. Willems G, Noack MJ, Inokoshi S, et al. Radiopacity of composites compared with human enamel and dentine. J Dent. 1991;19(6):362-365.
19. Joiner A. Review of the effects of peroxide on enamel and dentine properties. J Dent. 2007;35(12):889-896.
20. Lee YK, Lu H, Powers JM. Measurement of opalescence of resin composites. Dent Mater. 2005;21(11):1068-1074.
21. Lee YK, Lu H, Powers JM. Changes in opalescence and fluorescence properties of resin composites after accelerated aging. Dent Mater. 2006;22(7):653-660.
22. Cho MS, Yu B, Lee YK. Opalescence of all-ceramic core and veneer materials. Dent Mater. 2009;25;(6):695-702.
23. Yu B, Lee YK. Difference in opalescence of restorative materials by the illuminant. Dent Mater. 2009;25(8):1014-1021.
24. Yu B, Lee YK. Comparison of stabilities in translucency, fluorescence and opalescence of direct and indirect composite resins. Eur J Esthet Dent. 2013;8(2):214-225.
25. Panzeri H, Fernandes LT, Minelli CJ. Spectral fluorescence of direct anterior restorative materials. Aust Dent J. 1977;22(6):458-461.
26. Monsénégo G, Burdairon G, Clerjaud B. Fluorescence of dental porcelain. J Prosthet Dent. 1993;69(1):106-113.
27. McLaren EA. Luminescent veneers. J Esthet Dent. 1997;9(1):3-12.
28. Lee YK, Lu H, Powers JM. Effect of surface sealant and staining on the fluorescence of resin composites. J Prosthet Dent. 2005;93(3):260-266.
29. Sant’Anna Aguiar Dos Reis R, Casemiro LA, Carlino GV, et al. Evaluation of fluorescence of dental composites using contrast ratios to adjacent tooth structure: a pilot study. J Esthet Restor Dent. 2007;19(4):199-206,207.
30. Lee YK, Powers JM. Color changes of resin composites in the reflectance and transmittance modes. Dent Mater. 2007;23(3):259-264.
31. Lim YK, Lee YK. Fluorescent emission of varied shades of resin composites. Dent Mater. 2007;23(10):1262-1268.
32. Park MY, Lee YK, Lim BS. Influence of fluorescent whitening agent on the fluorescent emission of resin composites. Dent Mater. 2007;23(6):731-735.
33. Figueiredo C, Silva AM, Figueiredo A, Azenba ME. Fluorescence of dental composite resins. Experimental Pathology and Health Sciences. 2012;6(1):11-14.
34. Lee YK. Changes in the translucency of porcelain and repairing resin composite by the illumination. Dent Mater. 2007;23(4):492-497.
35. Ilie N, Hickel R. Correlation between ceramics translucency and polymerization efficiency through ceramics. Dent Mater. 2008;24(7):908-914.
36. Woo ST, Yu B, Ahn JS, Lee YK. Comparison of translucency between indirect and direct resin composites. J Dent. 2008;36(8):637-642.
37. Yu B, Lee YK. Differences in color, translucency and fluorescence between flowable and universal resin composites. J Dent. 2008;36(10):840-846.
38. Yu B, Lee YK. Influence of color parameters of resin composites on their translucency. Dent Mater. 2008;24(9):1236-1242.
39. Azzopardi N, Moharamzadeh K, Wood DJ, et al. Effect of resin matrix composition on the translucency of experimental dental composite resins. Dent Mater. 2009;25(12):1564-1568.
40. Kim SJ, Son HH, Cho BH, et al. Translucency and masking ability of various opaque-shade composite resins. J Dent. 2009;37(2):102-107.
41. Arimoto A, Nakajima M, Hosaka K, et al. Translucency, opalescence and light transmission characteristics of light cured resin composites. Dent Mater. 2010;26(11):1090-1097.
42. Kürklü D, Azer SS, Yilmaz B, Johnston WM. Porcelain thickness and cement shade effects on the colour and translucency of porcelain veneering materials. J Dent. 2013;41(11):1043-1050.
43. Wang F, Takahashi H, Iwasaki N. Translucency of dental ceramics with different thicknesses. J Prosthet Dent. 2013;110(1):14-20.
44. Ozakar Ilday N, Celik N, Bayindir YZ, Seven N. Effect of water storage on the translucency of silorane-based and dimethacrylate-based composite resins with fibres. J Dent. 2014;42(6):746-752.
45. Awad D, Stawarczyk B, Liebermann A, Ilie N. Translucency of esthetic dental restorative CAD/CAM materials and composite resins with respect to thickness and surface roughness. J Prosthet Dent. 2015;113(6):534-540.
46. Kim HK, Kim SH, Lee JB, Ha SR. Effects of surface treatments on the translucency, opalescence, and surface texture of dental monolithic zirconia ceramics. J Prosthet Dent. 2016:115(6):773-779.
47. Manojlovic D, Dramićanin MD, Lezaja M, et al. Effect of resin and photoinitiator on color, translucency and color stability of conventional and low-shrinkage model composites. Dent Mater. 2016;32(2):183-191.
48. Shiraishi T, Watanabe I. Thickness dependence of light transmittance, translucency and opalescence of a ceria-stabilized zirconia/alumina nanocomposite for dental applications. Dent Mater. 2016;32(5):660-667.
49. Lee YK, Powers JM. Metameric effect between resin composite and dentin. Dent Mater. 2005;21(10):971-976.
50. Kim SH, Lee YK, Lim BS, et al. Metameric effect between dental porcelain and porcelain repairing resin composite. Dent Mater. 2007;23(3):374-379.
51. Vanini L. Light and color in anterior composite restorations. Pract Periodontics Aesthet Dent. 1996;8(7):673-682.
52. Dietschi D. Layering concept in anterior composite restorations. J Adhes Dent. 2001;3(1):71-80.
53. Dietschi D, Ardu S, Krejci I. The silent revolution in dentistry. In: Exploring the Layering Concepts for Anterior Teeth. Berlin: Quintessence Publishing; 2004:235-250.
54. Dietschi D, Ardu S, Krejci I. A new shading concept based on natural tooth color applied to direct composite restorations. Quintessence Int. 2006;37(2):91-102.
55. Vichi A, Fraioli A, Davidson CL, Ferrari M. Influence of thickness on color in multi-layering technique. Dent Mater. 2007;23(12):1584-1589.
56. Niu Y, Ma X, Fan M, Zhu S. Effects of layering techniques on the micro-tensile bond strength to dentin in resin composite restorations. Dent Mater. 2009;25(1):129-134.
57. Özcan M, Pekkan G. Effect of delay in layering on the incremental adhesion of indirect dental composite resins. Int J Adhes Adhes. 2012;39:15-20.
58. Khashayar G, Dozic A, Kleverlaan CJ, et al. The influence of varying layer thicknesses on the color predictability of two different composite layering concepts. Dent Mater. 2014;30(5):493-498.
59. Brokos J, Mantas D, Lagouvardos P, Mountouris G. Digital photography in dentistry. Hellenic Stomatological Review. 2003;47:653-665.
60. Gordon P, Wander P. Specialized equipment for dental photography. Br Dent J. 1987;162(9):346-359.
61. Wang K, Kowalski EJ, Chung KC. The art and science of photography in hand surgery. J Hand Surg Am. 2014;39(3):580-588.
62. Sagawara Y, Saito K, Futaki M, et al. Evaluation of the optimal exposure settings for occlusal photography with digital cameras. Pediatric Dental. 2014;24(2):89-96.
63. Schaaf H, Streckbein P, Ettore G, et al. Standards for digital photography in cranio-maxillo-facial-surgery - Part II: Additional picture sets and avoiding common mistakes. J Craniomaxillofacial Surg. 2006;34(6):366-377.
64. Ettore G, Weber M, Schaaf H, et al. Standards for digital photography in cranio maxillo-facial-surgery – Part I: Basic views and guidelines. J Craniomaxillofacial Surg. 2006;34(2):65-73.
65. Jacobson A. Mastering dental photography. Am J Orthod Dentofacial Orthop. 2002;122(3):335.
66. Krieger G. Photography in dentistry: theory and techniques in modern documentation. Am J Orthod Dentofacial Orthop. 2012;142(4):568.
67. Keys LG, Agar JA. Documentation of maxillomandibular relationships during dental photography. J Prosthet Dent. 2002;87(4):466.
68. Tung OH, Lai YL, Ho YC, et al. Development of digital shade guides for color assessment using a digital camera with ring flashes. Clin Oral Investig. 2011;15(1):49-56.
69. Sascha H, Bazos P, Guardix JT, Naves LZ. Beyond visible: exploring shade interpretation. In: Duarte S, Jr., ed. Quintessence of Dental Technology. Hanover Park, IL: Quintessence Publishing; 2014:37:199-211.
70. Bazos P, Magne P. Bio-emulation: biomimetically emulating nature utilizing a histo- anatomic approach; visual synthesis. Int J Esthet Dent. 2014;9(3):330-352.
71. Shore V, Pardee AB. Fluorescence of some proteins, nucleic acids and related compounds. Arch Biochem BioPhys. 1956;60(1):100-107.
72. Armstrong WG. Ultra-violet spectrophotometric estimation of dentine protein in solution. Arch Oral Biol. 1962;7(6):771-772.
73. Booij M, ten Bosch JJ. A fluorescence compound in bovine dental enamel matrix compared with synthetic dityrosine. Arch Oral Biol. 1982;27(5):417-421.
74. Armstrong WG. Fluorescence characteristics of sound and carious human dentine preparations. Arch Oral Biol. 1963;8(2):79-90.
75. Matsumoto H, Kitamura S, Araki T. Autofluorescence in human dentine in relation to age, tooth type and temperature measured by nanosecond time-resolved fluorescence microscopy. Arch Oral Biol. 1999;44(4):309-318.
76. Lee YK. Fluorescence properties of human teeth and dental calculus for clinical applications. J Biomed Opt. 2015;20(4):040901.
77. Armstrong WG. The presence of ultra violet absorbing material and its relation to fluorescence 'quenching' effects in carious dentine. Arch Oral Biol. 1963;8(2):223-231.
78. Foreman PC. The excitation and emission spectra of fluorescent components of human dentine. Arch Oral Biol 1980;25(10):641-647.
79. Hafström-Björkman U, Sundström F, ten Bosch JJ. Fluorescence in dissolved fractions of human enamel. Acta Odontol Scand. 1991;49(3):133-138.
80. Bachmann L, Zezell DM, da Costa Ribeiro A, et al. Fluorescence spectroscopy of biological tissues—a review. Appl Spectrosc Rev. 2006;41(6):575-590.
81. Ruttermann S, Ritter J, Raab WH, et al. Laser-induced fluorescence to discriminate between a dental composite resin and tooth. Dent Mater. 2007;23(11):1390-1396.
82. Zhang L, Nelson LY, Seibel EJ. Red-shifted fluorescence of sound dental hard tissue. J Biomed Opt. 2011;16(7):071411.
83. Queiroz RS, Bandeca MC, Calixto LR, et al. Influence of the light-curing unit, storage time and shade of a dental composite resin on the fluorescence. Laser Phys. 2010;20(7):1647-1653.
84. Kim HE, Kim BI. An in vitro comparison of quantitative light-induced fluorescence-digital and spectrophotometer on monitoring artificial white spot lesions. Photodiagnosis Photodyn Ther. 2015;12(3):378-384.
85. Meller C, Klein C. Fluorescence of composite resins: A comparison among properties of commercial shades. Dent Mater J. 2015;34(6):754-765.
86. Lee YK, Yu B. Measurement of opalescence of tooth enamel. J Dent. 2007;35(8):690-694.
87. Schmeling M, Maia HP, Baratieri LN. Opalescence of bleached teeth. J Dent. 2012;40(suppl 1):e35-e39.
88. Pecho OE, Ghinea R, do Amaral EA, et al. Relevant optical properties for direct restorative materials. Dent Mater. 2016;32(5):e105-e112.
89. Pop-Ciutrila IS, Ghinea R, Colosi HA, Dudea D. Dentin translucency and color evaluation in human incisors, canines, and molars. J Prosthet Dent. 2016;115(4):475-481.
90. Li Q, Xu BT, Li R, Wang YN. Spectrophotometric comparison of translucent composites and natural enamel. J Dent. 2010;38(suppl 2):117-122.
91. Mikhail SS, Schricker SR, Azer SS, et al. Optical characteristics of contemporary dental composite resin materials. J Dent. 2013;41(9):771-778.
92. Wyszecki G, Stiles WS. Color Science: Concepts and Methods, Quantitative Data and Formulae. 2nd ed. New York, NY: Wiley-Interscience; 2000.
93. Craig RG, Powers JM. Restorative Dental Materials. 11th ed. St. Louis, MO: Mosby; 2002.
94. Marcus RT. The measurement of color. In: Nassau K, ed. Color for Science, Art and Technology. Amsterdam, Netherlands: Elsevier; 1998:31-96.
95. O’Brien WJ. Double layer effect and other optical phenomena related to esthetics. Dent Clin North Am. 1985;29(4):667-672.
96. Leow ME, Ng WK, Pareira BP, et al. Metamerism in aesthetic prostheses under three standard illuminants-TL84, D65 and F. Prosthet Orthot Int. 1999;23(2):174-180.
97. Thornton WA. How strong metamerism disturbs color spaces. Color Res Appl. 1998;23(6):402-407.
98. Paravina RD, Johnston WM, Powers JM. New shade guide for evaluation of tooth whitening—colorimetric study. J Esthet Restor Dent. 2007;19(5):276-283.
99. Kourtis SG, Tripodakis AP, Doukoudakis AA. Spectrophotometric evaluation of the optical influence of different metal alloys and porcelains in the metal-ceramic complex. J Prosthet Dent. 2004;92(5):477-485.
100. Eves MG. Shade selection and value control. J Dent Technol. 2000;17(1):11-17.
101. Culpepper WD. A comparative study of shade-matching procedures. J Prosthet Dent. 1970;24(2):166-173.
102. van der Burgt TP, ten Bosch JJ, Borsboom PCF, Kortsmit WJPM. Color measuring devices. Dental Abstracts. 2008;53(5):255-256.
103. Chu SJ, Trushkowsky RD, Paravina RD. Dental color matching instruments and systems. Review of clinical and research aspects. J Dent. 2010;38(suppl 2):e2-e16.
104. Sarafianou A, Kamposiora F, Papavasiliou G, Goula H. Matching repeatability and interdevice agreement of 2 intraoral spectrophotometers. J Prosthet Dent. 2012;107(3):178-185.
105. Papazoglou E, Brokos J, Mountouris G. A method to capture polarized digital dental photographs. Italian Journal of Operative Dentistry. 2006;IV(1):45.